This Is The First Artificial Pancreas
Oct 03, 2013 00:12
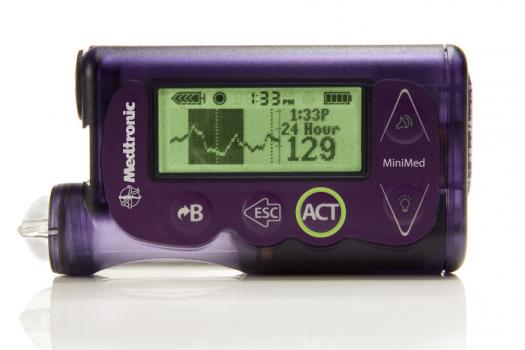
The first artificial pancreas is here and has been approved by the U.S. FDA. It was designed by Minneapolis-based medical tech company Medtronic and is a wearable little gadget that stops insulin delivery automatically when glucose levels get too low. Will we see this take off?
The hormone insulin controls blood sugar levels which are normally produced in the body by the pancreas. Type 1 diabetics and sometimes Type 2 patients, find that their pancreas just doesn't make any insulin and their bodies can't regulate blood sugar levels.
In traditional insulin pumps, wearers have to monitor their blood sugar levels and manually program the pump to deliver insulin. This one however will monitor blood sugar for you and deliver the appropriate amount accordingly.
Medtronic's MiniMed 530G system can detect up to 93 percent of hypoglycemia (low blood sugar) episodes, and will sound an alarm to wake you up if your blood sugar gets too low. If you don't respond to it, the system will shut off insulin delivery for two hours to stave off low blood sugar levels.
But Medtronic has got a warning letter from the FDA related to the manufacturing processes of their Paradigm Insulin Infusion Pumps (which are used in this system) at their facility in Northridge, Calif.
The pumps have been recalled in June because they were malfunctioning and delivering either too much or too little insulin.
The company said in a press release accompanying the product approval that it had "already addressed many of the observations noted in the warning letter and is committed to resolving the remaining observations as quickly as possible."
[Singularity Hub]